The National Agency for Food and Drugs Administration and Control, NAFDAC, has alerted the public to the fake malaria drug in circulation.
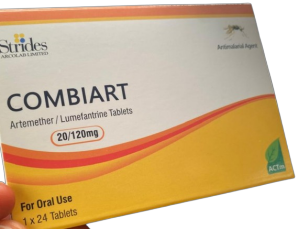
According to the agency, the counterfeit Combiart Dispersible Tablet 20/120mg anti-malaria drug is currently in circulation in the country.
This was contained in a statement by the Agency in a post on its X handle on Thursday.
NAFDAC stated that it had alerted its offices nationwide to begin the immediate mop-up of the fake malaria drug. It gave the manufacture of the drug as Strides Arcolab Limited, based in India.
The statement read, “This product was discovered in the FCT and Rivers State, during surveillance activities conducted by officers of the Post Marketing Surveillance Directorate of NAFDAC.
“The Laboratory report of the analysis carried out on the product revealed that it contained Zero Active Pharmaceutical Ingredients. The product was also observed to have two different date markings. The NAFDAC database of registered products has confirmed that the product license has expired. And the NAFDAC Registration Number on it is wrong and not for the product.
“The Artemether and Lumefantrine combination belongs to a group of medicines known as antimalarials. It treats malaria; a red blood cell infection transmitted by the bite of a mosquito. However, this medicine is not used to treat severe or complicated malaria.”
NAFDAC, therefore, warned that counterfeit or falsified medicines endanger people’s health. This is because they do not comply with regulatory standards. Which means the safety, quality, and efficacy of these products are not ensured.
The agency added that the use of counterfeit medicines often fails to effectively treat diseases or conditions. Thereby, leading to serious health consequences, including death.
NAFDAC added that the the product batch number is 7225119 with a NAFDAC registration number of A11-0299.
It noted that the product’s manufacturing dates are June 2023, and February 2023. The expiry dates are May 2026, and June 2026
The agency gave the manufacturer’s name and address as Strides Arcolab Limited, 36/7, Suragajakkanahalli, Indlavadi Cross, Anekal Taluk, Bangalore- 562 106, India.
The statement read in part, “All NAFDAC Zonal directors and State Coordinators have been directed to carry out surveillance. And to mop up the counterfeit products within the Zones and States.
“Importers, distributors, retailers, healthcare professionals, and caregivers are hereby advised to exercise caution and vigilance within the supply chain. This is to avoid the importation, distribution, sale, and use of counterfeit products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.
“Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, NAFDAC on 0800-162-3322 or via email: sf.alert@nafdac.gov.ng.
“Similarly, healthcare professionals and patients are also encouraged to report adverse events or side effects related to the use of medicinal products or devices to the nearest NAFDAC office. Or through the use of the E-reporting platforms available on the NAFDAC website www.nafdac.gov.ng. Or via the Med- safety application available for download on android and IOS stores or via e-mail on pharmacovigilance@nafdac.gov.ng,” it said.
NAFDAC noted that the notice will be uploaded to the World Health Organization Global Surveillance and Monitoring System.
Follow us for more news on our WhatsApp News Channels @
https://whatsapp.com/channel/0029VaC505jB4hdZ5Yx9g82U
![]()